Transitioning small batch manufacturing to large-scale production.
Operational Velocity worked closely with a diagnostic start-up in northern California. The company had developed an antigen COVID-19 test that provided results with an accuracy equivalent to PCR tests. They were seeking support with the transition to manufacturing and commercialization. At the start of the project, the diagnostic company had only built in small batches and without all design controls in place.
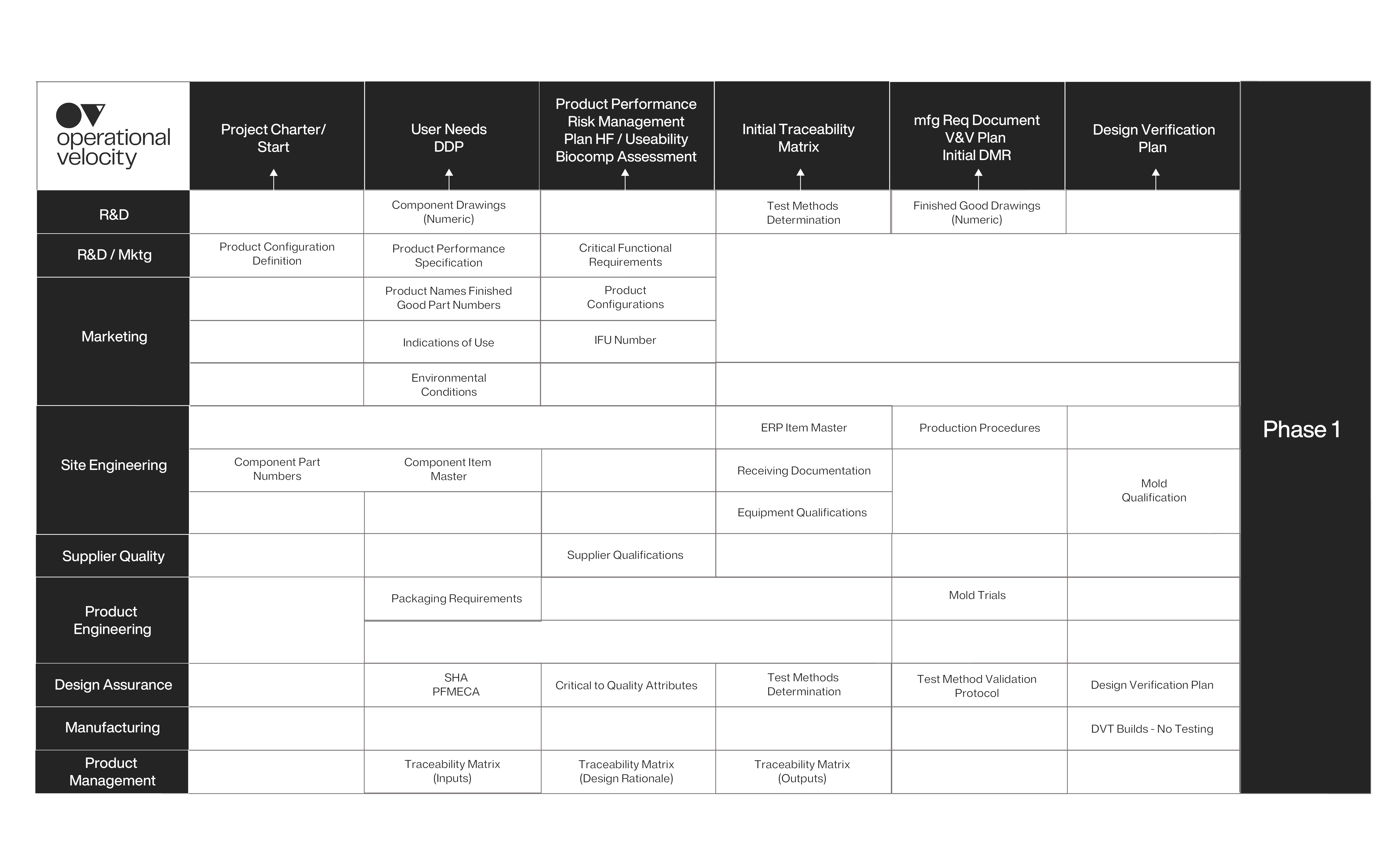
Operational Velocity provided an initial assessment to identify where the technical documentation had strengths and opportunities (see below). Collaborating and supplementing experience with the manufacturing engineering team, Operational Velocity performed product cost modeling and manufacturing scenarios analysis, identifying hidden risks in drawings, procedures, and supply/quality agreements to deliver on phase II deliverables. This information helped identify initial profit margins, cash management requirements, and opportunities for future cost savings.
Operational Velocity authored, integrated, and coordinated implementation of procedures and templates for validation and design transfer into the existing QMS to mature existing procedures for both manufacturing and quality. As the emergency use authorization (EUA) pathway allowed for commercialization without all the quality system requirements in place, Operational Velocity helped navigate and provide documentation and deliverables that met regulatory requirements and successful EUA approval.
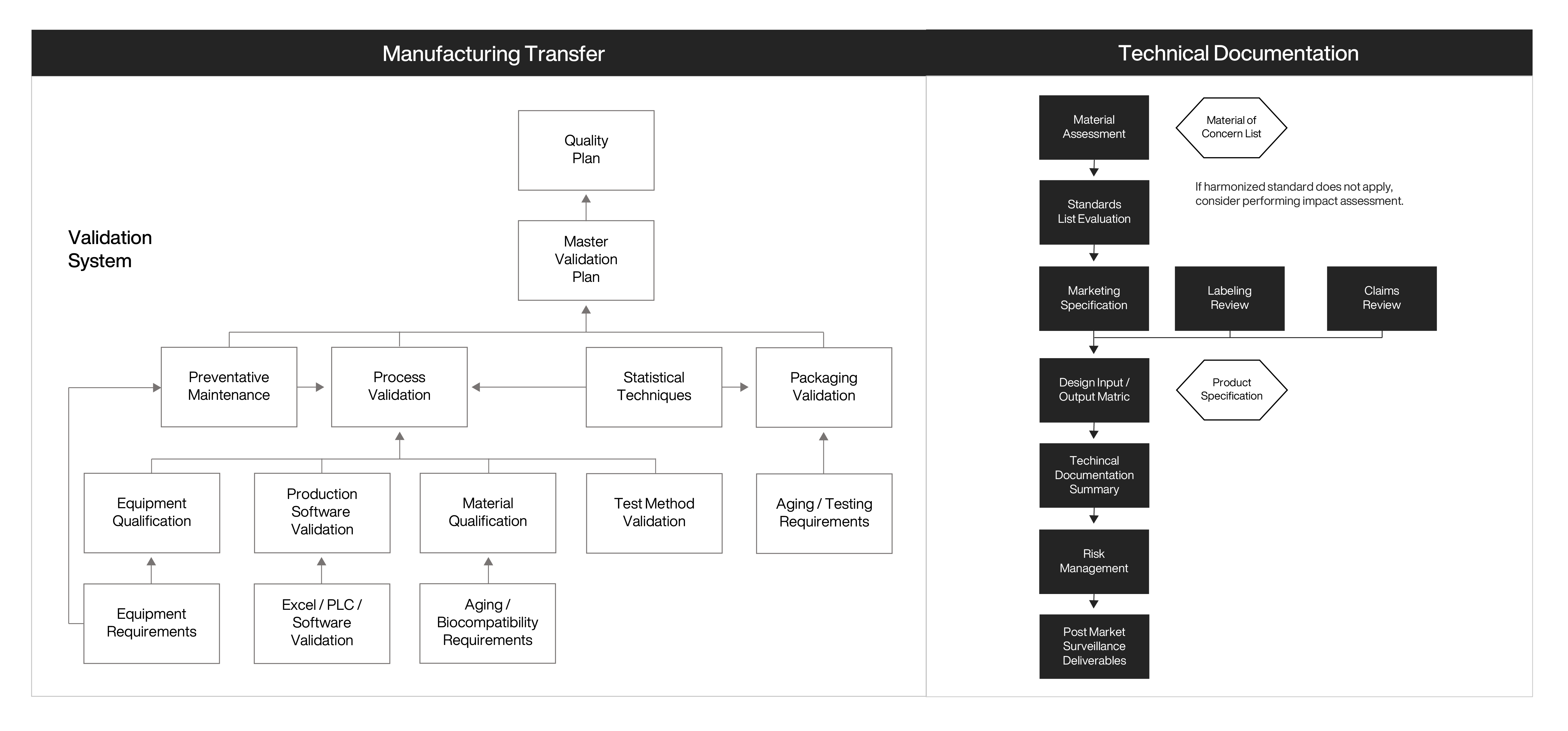
Continuing beyond the initial assessment and support, Operational Velocity coordinated and provided oversight in the transition to a contract manufacturer. By providing direct feedback, authoring changes, and mentoring engineers, Operational Velocity improved manufacturing and technical documentation to avoid issues when transitioning to a contract manufacturing. Once equipment qualifications and process validation activities started as planned, the customer began their commercialization journey and IPO activities.
Subsequent work with the government teams as subject matter experts for the commercialization of products affirmed Operational Velocity’s expertise in transitioning small batch manufacturing to large-scale production.

Operational Velocity
109 N Maple St, Unit J
Corona, CA 92878-3298
951 475 7038 | 949 445 0532
© 2024 Operational Velocity